
A research team led by Prof. HUANG Chengdong from the University of Science and Technology of China of the Chinese Academy of Sciences (CAS), user of he Steady High Magnetic Field Facility (SHMFF) at the Hefei Institutes of Physical Science of CAS, has identified a novel mechanism by which intrinsically disordered regions (IDRs) remotely regulate protein function—acting as molecular "on-off" switches through modulation of conformational entropy, without direct contact with the protein' s functional domain.
The findings, published in Nature Structural & Molecular Biology, were realized by SHMFF, including the SM3 superconducting magnet and a state-of-the-art 850 MHz solution Nuclear Magnetic Resonance (NMR) spectrometer.
IDRs are widespread in proteins but have long been dismissed as non-functional due to their lack of stable structure—often regarded as "useless tails."
In this study, the researchers used the molecular chaperone Sgt2 as a model system and applied high-resolution solution NMR combined with biochemical techniques to dissect its regulatory mechanism. The findings showed that Sgt2' s auto-inhibition is not governed by its structured TPR domain or the linker, but instead by the specific amino acid sequence of its C-terminal IDR. Strikingly, modifying this IDR sequence abolished the protein's ability to self-regulate, suggesting that the IDR functions as a critical regulatory element rather than evolutionary residue.
Contrary to conventional models that rely on conformational changes to control protein activity, this study demonstrated that Sgt2's switching mechanism is entirely driven by internal molecular dynamics—specifically, changes in conformational entropy.
When the IDR is intact, the protein remains in a highly dynamic state with high entropy, effectively locking the functional domain and preventing activity. Upon deletion of the IDR or substrate binding, the molecule becomes more ordered and rigid, releasing the inhibition and activating the protein.
This discovery challenges the conventional belief that protein regulation requires structural interaction or conformational change, offering a new conceptual framework for designing dynamic, controllable molecular switches, according to the team.
This study was supported by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, and the Ministry of Education Key Laboratory for Membrane-less Organelles and Cellular Dynamics, among others.
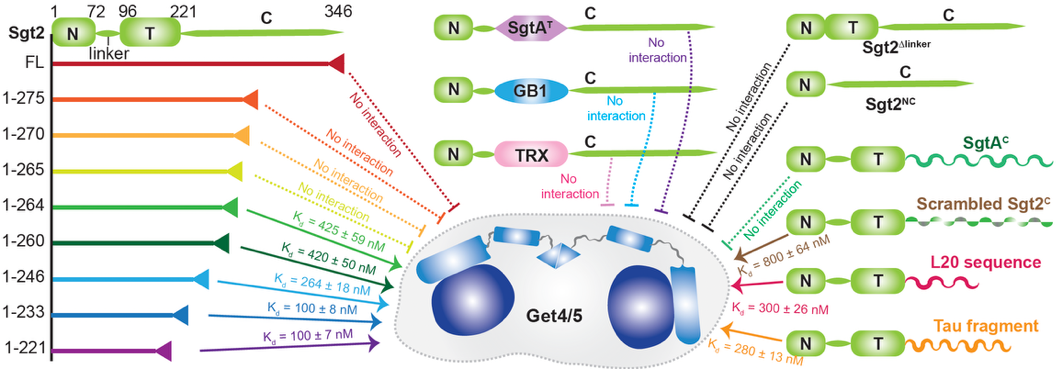
Fig. 1. Sgt2 auto-inhibition is determined by the specific sequence of the C-terminal IDR and is independent of the middle TPR domain or other protein fragments. (Image by JI Tuo)
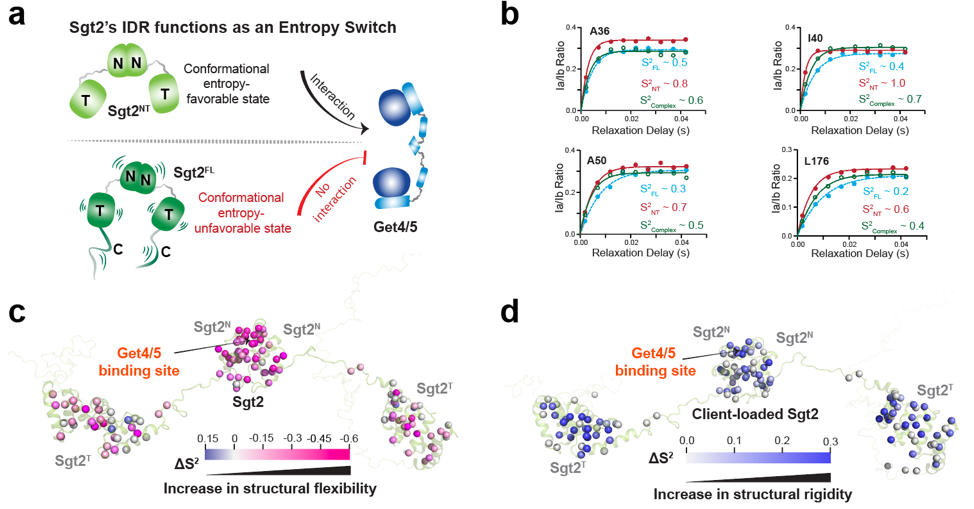
Fig. 2. Sgt2's IDR enables remote auto-inhibition and activation without direct contact by regulating molecular conformational entropy, thereby dynamically switching the protein's binding capacity. (Image by JI Tuo)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

